Safe use of workplace chemicals
Donald E. Larson
American Polywater Corp.
While doing mundane tasks at home or in the workplace, we are frequently exposed to hazardous chemicals. We must learn how to use such chemicals safely to minimize personal risk. But how can we learn about the problems and hazards of a particular material we use in the workplace, and what can we do about them? Here we will discuss chemical hazards and safety and, specifically, the toxicology of workplace chemicals.
Toxicology is simply the science of poisons. If you look at toxicological data for baking soda, table salt, and cyanide, you may be surprised to find that all of them have lethal doses. However, cyanide is extremely toxic and can be fatal at very low exposure levels. The two food materials have very-low-level toxicity, and large quantities are required for adverse systemic effects. It is important to know the degree or severity of toxicity in order to assess the hazard and develop safe material handling procedures.
Another important factor is the way one may be exposed to a specific workplace chemical. Most of us think of poisons as being eaten or ingested (called oral exposure). However, toxic materials can also enter the human body through the skin (dermal exposure) or via the lungs (inhalation). In industrial situations, inhalation and dermal exposures are often the primary concerns. Most workers know they should not eat workplace materials but may not think about problems that result from smelling or touching them.
Where to start
Information on exposure to and severity of workplace chemicals should be on the material safety data sheet (msds) for the specific chemical substance. This sheet is available from the chemical`s manufacturer and will provide information on toxicity, exposure, and first-aid. It also contains information on fire hazard, chemical incompatibilities, disposal, and shipping.
Unfortunately, some msdss provide only marginally adequate information on procedures that deal with the safe use of chemicals. A thorough msds, on the other hand, contains considerable information, but it does not interpret it for your situation and application. So, how can you interpret and use the msds?
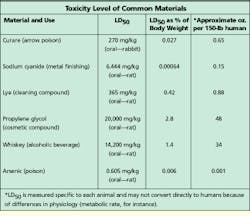
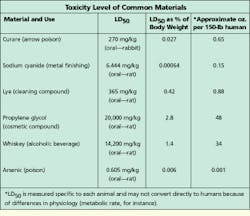
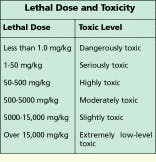
Because a thorough msds provides information that covers a variety of product uses or misuses, some of the terminology can be scary. One term often found on an msds is LD50, which stands for the lethal dose required to produce a 50% death rate in test animals--that is, the quantity of material which--when administered orally, dermally, or intravenously--would result in the death of 50% of a group of test animals such as rats, mice, or guinea pigs. LD50 data is presented as a weight of the chemical substance to the weight of the animal, most commonly in milligrams of substance per kilogram of body weight (mg/kg).
LD50 limits for some familiar chemicals as well as a common use for the substance are provided in the table. LD50 levels vary by a factor of millions. This type of data is one of the ways to determine degree of toxicity. LD50 data becomes more understandable when you look at what the animal data means in terms of human exposure.
Based on this kind of information, a 150-pound person would have to ingest several quarts of a chemical to reach the 15,000-mg/kg level, which represents an extremely low-level toxicity.
Because their major component is water, for example, most cable-pulling lubricants have extraordinarily low toxicity. Ingestion of up to 50% of body weight, or 10 to 20 gallons, is necessary for such compounds to reach a level that produces systemic effects.
Dermal toxicity
Dermal toxicity can be quantified in the same way as ingestion, via LD50, but the quantity that must be determined is the amount of material that must be absorbed through the skin to produce toxic effects. Fortunately, the absorption of many substances through thick, exposed skin, such as that of the hands, is rather slow. There are, however, notable exceptions, including certain pesticides and steroids.
A different aspect of skin exposure is sensitivity or allergic reaction. Such reactions, which are specific to an individual, involve exposure to quantities much smaller than those necessary for systemic toxic effects. Almost any material can produce an allergy in a hypersensitive person. Skin-patch tests are used to indicate the general skin irritation potential of various materials.
Inhalation
Exposure to airborne dusts, mists, fumes, and vapors is difficult to determine and control in a construction environment. Because the primary evaporating component is water, inhalation exposure is not a consideration in most uses of cable lubricants. However, the cleaning solvents frequently used in cabling work do have evaporating solvents, which present a potential respiratory- or inhalation-exposure problem.
The inhalation version of the oral lethal dose is called the LC50--lethal concentration fifty--or the concentration required to produce a 50% death rate in a test animal. LC50 data involves not only the concentration of the substance in the air--usually in milligrams per cubic meter (mg/m3) or parts per million (ppm)--but also exposure time, animal type, and other factors.
Permissible exposure limits
Because inhalation is such a common industrial exposure, hygiene groups and government agencies such as the Occupational Safety and Health Administration (Washington, DC) and the National Institute of Occupational Safety and Health (Washington, DC) have developed a set of airborne exposure limits called threshold limit values (tlvs), or permissible exposure limits. These limits are given in ppm of air or parts per hundred million (pphm) of air. The most frequently used tlv limits are called time-weighted averages and represent the maximum recommended airborne concentration under which most people can work for an eight-hour day without adverse health effects.
tlvs for solvent cleaners and degreasers can be found on their msdss. But what if a cleaning solvent has no listed tlv? Does this mean that unlimited vapor exposure is safe? Obviously not. "No listed tlv" means that there is not enough information available for industrial-hygiene experts to establish a tlv, or a safe working level. Without such guidance, we must look for other data to determine safe exposure levels and potential respiratory needs. Data such as LC50 animal tests or tlvs from chemically similar materials might be considered.
Many people think that a material with a tlv of 500 ppm is safer than one with a tlv of 100 ppm, but this is not necessarily true. For instance, if the solvent with the tlv of 500 ppm evaporates quickly, vapor levels well above 500 ppm could easily occur in a vault or confined area. If a solvent with a tlv of 100 ppm is slow to evaporate, the airborne concentration in the vault may never reach 100 ppm. The safety of a cleaning solvent depends not only on the tlv, but also on how much of the material evaporates, in what type of area it is used, and with what kind of ventilation.
Does the nose know?
Another frequently asked question is whether smell will tell us when the airborne concentration of a solvent exceeds its tlv. Unfortunately, no. Some cleaning solvents may have strong, irritating odors well below their tlv level. Others, however, may have very little odor at concentrations well above their permissible exposure limits.
One way American Polywater Corp. has addressed the problem of vapor inhalation of cleaning solvents is to conduct its own tests of vapor concentration in a confined work area. For example, the company offers with its HydraSol Cable Gel Remover a pouch containing a lint-free towel saturated with water-based HydraSol Cleaner (HS-1). By determining the rate and amount of solvent evaporation from the HS-1 package, American Polywater has been able to develop a computer program to determine the vapor concentration. In one such simulation, the vapor concentration from a single HydraSol towelette reached about 10 ppm in a vault of 512 cubic feet with a ventilation rate of 64 cu. ft. per minute and a temperature of 60oF. This concentration is less than 5% of the suggested tlv for the solvent of 500 ppm. Using such a package allows workers to control their exposure to solvent vapors, which is impossible when using bulk cleaners such as aerosol sprays and gallon drums.
Editor`s Note: There is additional important information about chemical toxicology and safe chemical use that has not been covered in this article. Your company`s industrial hygienist is the expert in this area and should be consulted with specific problems and questions.
Donald E. Larson is national sales manager of American Polywater Corp. (Stillwater, MN), which manufactures chemicals and materials used in telecommunications construction. This column was excerpted from his company`s technical newsletter, TeleTopics.